The Science Of Pylopass | L. Reuteri DSM 17648
Proven results within 56 days.
Natural Solution
Side-effect free. Pylopass is transient, meaning it goes in crowds the 'bad' stomach bacteria and removes it via the stool.
Clinically Proven
L. Reuteri DSM 17648 has been well-research with 4 peer-reviewed RCT's. Pylopass can be used from ages 9+.
Safe Alongside Antibiotics
Pylopass alongside Quadruple therapy improved eradication and showed improvements in symptoms within 28 days.
How Pylopass Works.
The Lactobacillus strain DSM17648 (Pylopass) attaches directly to the stomach bacteria. It 'crowds' around the bacteria and removes it from the stomach and out of the body via your stool.
This is why Pylopass has become a safe, recognised and effective natural alternative for this specific stomach bacteria.
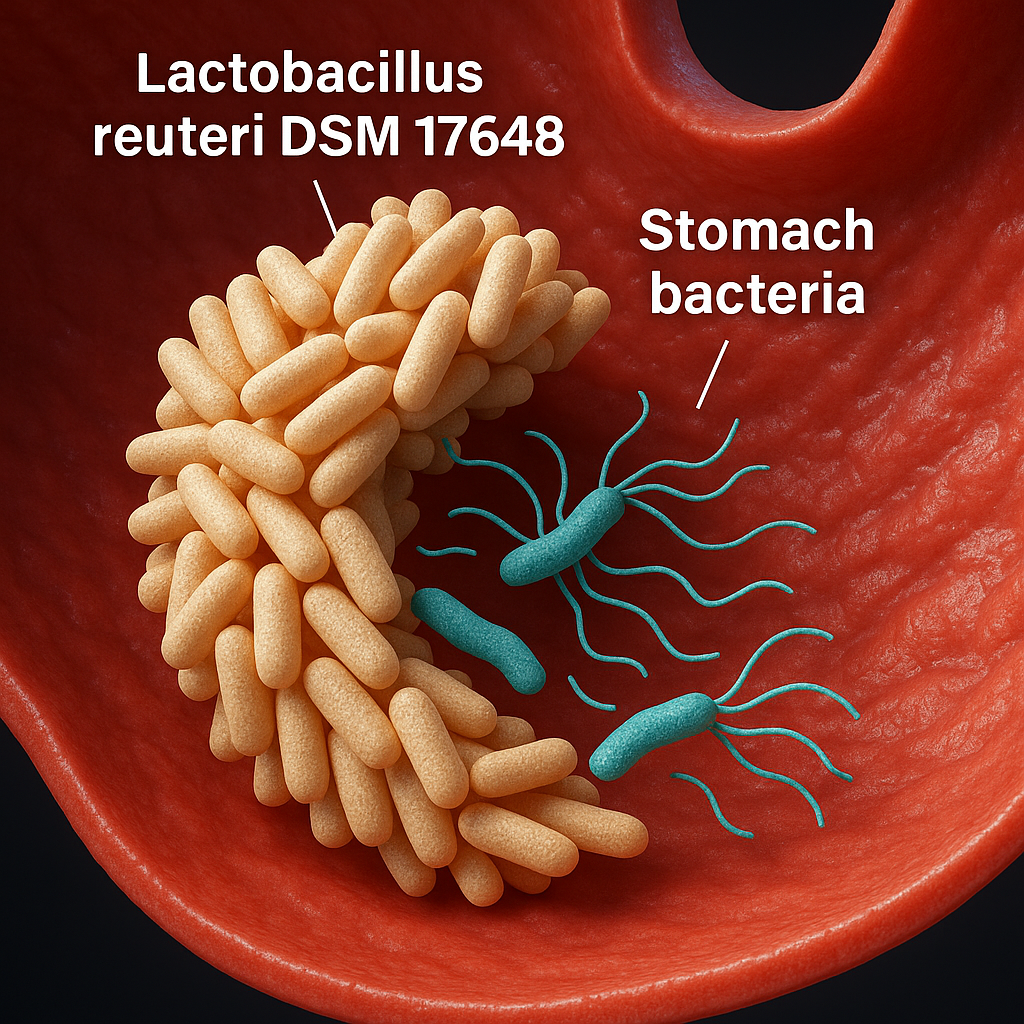
62.5% of participants experienced a measurable decrease in bacteria load.
Long-Lasting Results.
Whether you think you might have it or definitely have it, Pylopass is a safe, natural, and side-effect free alternative.
Lactobacillus reuteri offers a unique and new approach to addressing this specific stomach bacteria, which affects 50% of the global population.



